-
PDF
- Split View
-
Views
-
Cite
Cite
Ali Nasrolahi, Stephanie B. Stratil, Katharina J. Jacob, Martin Wahl, A protective coat of microorganisms on macroalgae: inhibitory effects of bacterial biofilms and epibiotic microbial assemblages on barnacle attachment, FEMS Microbiology Ecology, Volume 81, Issue 3, September 2012, Pages 583–595, https://doi.org/10.1111/j.1574-6941.2012.01384.x
Close - Share Icon Share
Abstract
Effects of epibiotic bacteria associated with macroalgae on barnacle larval attachment were investigated. Eight bacterial isolates obtained from samples of three macroalga species were cultured as monospecies bacterial films and tested for their activity against barnacle (Amphibalanus improvisus) attachment in field experiments (Western Baltic Sea). Furthermore, natural biofilm communities associated with the surface of the local brown alga, Fucus vesiculosus, which were exposed to different temperatures (5, 15 and 20 °C), were harvested and subsequently tested. Generally, monospecies bacterial biofilms, as well as natural microbial assemblages, inhibited barnacle attachment by 20–67%. denaturing gradient gel electrophoresis fingerprints showed that temperature treatment shifted the bacterial community composition and weakened the repellent effects at 20 °C. Repellent effects were absent when settlement pressure of cyprids was high. Nonviable bacteria tended to repel cyprids when compared to the unfilmed surfaces. We conclude that biofilms can have a repellent effect benefiting the host by preventing heavy fouling on its surface. However, severe settlement pressure, as well as stressful temperature, may reduce the protective effects of the alga's biofilm. Our results add to the notion that the performance of F. vesiculosus may be reduced by multiple stressors in the course of global warming.
Introduction
In marine habitats, nearly all submerged natural and artificial surfaces become covered by biofilm. Microorganisms, such as bacteria and diatoms, are among the first colonizers (Wahl, 1989) that can substantially change the physical and chemical properties of the substratum (Characklis & Cooksey, 1983), making it suitable or unsuitable for colonization by invertebrate larvae (reviewed by Qian et al., 2007). Inhibitory effects of biofilms on larval settlement have been documented in many studies (e.g. Olivier et al., 2000; Lau et al., 2003; Dobretsov & Qian, 2006; Rao et al., 2007; Ganesan et al., 2010). However, inductive or neutral effects have also been reported (e.g. Wieczorek et al., 1995; Harder et al., 2002; Ganesan et al., 2010). The inhibitory effect of biofilms has been mainly attributed to their bacterial components (Maki et al., 1988; Holmström et al., 1992; Avelin Mary et al., 1993; Anil & Khandeparker, 1998; Lau & Qian, 2000; Khandeparker et al., 2002, 2003; Dobretsov & Qian, 2006). Previous studies have shown that bacterial surface chemistry, microtopography and a range of bacterial products from small-molecule metabolites to high-molecular-weight extracellular polymers mediate cyprid settlement (reviewed by Qian et al., 2007). It is further known that biofilm community composition, which can be a determinant for larval attachment, can be affected by environmental factors, such as temperature, and that these altered biofilm communities can change the settlement behaviour of benthic larvae (Lau et al., 2005). Structural and chemical cues (e.g. 6, 9-heptadecadiene and 12-octadecenoic acid, Hung et al., 2007) from the natural biofilm community play an important ecological role in the natural environment of barnacle larvae by aiding in the search for suitable habitat. For instance, more barnacle larvae attach to substrate with biofilms from environments that are favourable to barnacle recruitment than to substrate with biofilms from less favourable environments (Hung et al., 2007). Amphibalanus amphitrite cyprids from the mid-intertidal can also distinguish between biofilms from different tidal ranges and settle preferentially on intertidal biofilms compared to subtidal biofilms and unfilmed surfaces (Qian et al., 2003). Furthermore, the attachment behaviour of the barnacle species A. amphitrite and Balanus trigonus was shown to depend on the temperature at which biofilms were grown, with more cyprids attaching to biofilms formed at higher temperatures (> 23 °C) than to ones formed at 16 °C (Lau et al., 2005).
Most studies on interactions between biofilms and invertebrate colonizers have focused their experiments on biofilms grown on nonliving substrata (e.g. Qian et al., 2003; Hung et al., 2007). In the marine system, however, also living surfaces such as macroalgae thalli are potentially suitable substratum for invertebrate larvae in competition for space. Uncontrolled colonization of epibiotic microorganisms on the macroalga usually has detrimental effects, for example, by shading its surface and thus reducing its growth rate (Rohde et al., 2008). To counteract this, the brown macroalga, Fucus vesiculosus, can produce secondary metabolites that directly repel invertebrate larvae (Brock et al., 2007; Lachnit et al., 2010). Lachnit et al. (2010) suggested that F. vesiculosus can also indirectly protect its surface from unwanted colonizers via the regulation of a beneficial epibiotic bacterial biofilm community that produces repellent substances. If a potentially beneficial epibacterial community undergoes a shift in its composition (via direct stress and/or via indirect stress on the alga), the attachment of macrofouler larvae could in turn be affected. The immensely important function of biofilms in attachment processes of barnacles (and other invertebrate larvae) and the potential interaction with their macroalgal host led us to investigate the effect of biofilms composed of bacteria epibiotic on macroalgae on the number of attached Amphibalanus simprovisus cyprids in field assays. Specifically, our objectives were (1) to test the effect of monospecies bacterial biofilms originally isolated from macroalgal hosts on the attachment of barnacle larvae, (2) to investigate how effective these monospecies biofilms are under natural settlement pressures, (3) to test the effect of water-soluble chemical compounds from a natural assemblage of microorganisms detached from their brown macroalgal host, F. vesiculosus, on the attachment of barnacle larvae and (4) to test the indirect effects of environmental abiotic stress (i.e. temperature) via a shift in the epibiotic bacterial community composition on the attachment of cyprids.
Materials and methods
Monospecies bacterial films
Monospecies bacterial films were developed from eight bacterial strains, which had been isolated from three locally (Kiel Bight, Western Baltic Sea) common macroalgal species (Schories et al., 2009), and were tested for their effects on the attachment of cyprids in field assays. According to 16S rRNA gene sequences, bacterial isolates affiliated to Pseudoalteromonas mariniglutinosa (JQ867499), Pseudoalteromonas tunicata (JQ867503) and Shewanella baltica (JQ867502); Bacillus foraminis (JQ867498) isolated from the brown macroalga F. vesiculosus (Linneaeus 1753); Ulvibacter litoralis (JQ867505) and Photobacterium halotolerans (JQ867504) isolated from Fucus serratus (Linneaeus 1753); Pseudoalteromonas arctica (JQ867501) and Shewanella basaltis (JQ867500) isolated from the red alga Polysiphonia stricta (Dillwyn) Greville 1824. The 16S rRNA gene sequences of the bacterial isolates were deposited in GenBank under the accession numbers JQ867498–JQ867505.
Bacterial cultures were grown in sterile nutrient broth [5 g peptone + 3 g yeast in 1 L of filtered seawater (FSW)]. Each strain was inoculated to individual culture flasks containing 50 mL of sterile nutrient broth prepared with 0.22-μm FSW and incubated for 45–48 h at 20 °C. Prior to the assays, the optical density (OD) of the bacterial cultures was determined with a Beckman Du 650 spectrophotometer at a wavelength of 600 nm, using pure medium as blank. All working cultures were adjusted to an OD of 1.5. Cultures were diluted to the mentioned OD with sterile medium when necessary.
Formation of monospecies bacterial films
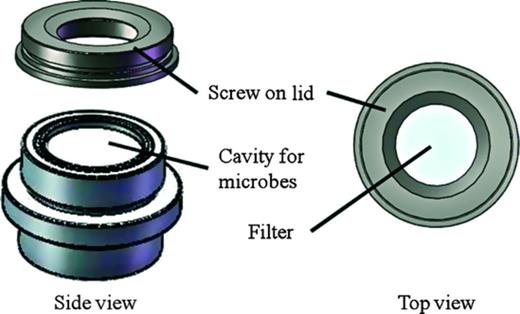
Bacteria were harvested from cultures and adjusted to around 108 cells mL−1 by turbidimetry. About 0.2-μm polycarbonate filters (Sartorius, Germany) with a diameter of 47 mm served as settlement substratum for the bacteria. The sterile filters were placed onto a specially constructed sterile apparatus and fixed tightly with a screw-on lid that partially covered the filter (Fig. 1). This reduced the filter diameter to 35 mm and resulted in an effective attachment area of 9.62 cm2 onto which 2 mL of bacterial suspension was pipetted. After 4 h of incubation under a sterile hood at room temperature (ca. 20 °C) under continuous light, the filters were gently rinsed with autoclaved FSW to remove unattached bacteria. Cells attached to the filter surface will be referred to as monospecies bacterial films hereafter and were formed as described previously for all experiments.
Apparatus used in the cyprid attachment field assays. Natural biofilms were placed in the cavity underneath the filter during assays, monospecies bacteria formed a film on top of the filter, which in all cases was used as attachment substratum for cyprids. The area available for attachment was 9.62 cm2. The screw-on lid tightly sealed the filter cavity and held the filter in place. One apparatus was used for one replicate. Individual apparatuses were fixed to a PVC frame and deployed in the Kiel Fjord. Courtesy of Ralf Schwarz.
Preparation of nonviable bacterial films
Monospecies bacterial films were pretreated with formaldehyde to generate nonviable films. Bacterial films were covered with 2 mL of formaldehyde (3.7% in FSW) for 30 min and were subsequently rinsed five times with autoclaved FSW (Lau et al., 2003). Control dishes did not contain any bacterial film but were treated with formaldehyde following the same procedure as for the bacterial films.
Enumeration of bacterial cells
Before initiating the attachment bioassay, the density of bacteria on reference filters was enumerated using epifluorescence microscopy (Zeiss Axio Scope A1 fluorescence microscope). After 4 h of incubation, the bacterial films on the filters were fixed with 3.7% formaldehyde in FSW for 15 min and subsequently stained with 0.2% (v/v) 4,6-diamidino-2-phenylindole (DAPI; Invitrogen) for 15 min at room temperature. For bacterial enumeration, cell numbers in five randomly chosen fields of view were counted at ×1000 magnification. In the fifth experiment, we did not enumerate bacteria. Microscopic examination of the filters, however, showed a similar coverage of the filters by bacterial cells as in the other experiments, indicating that settlement of bacteria was successful. The relationship between bacterial density and attached cyprids was analysed. Data from experiment II were excluded from analysis because there was no effect of bacterial films on cyprid attachment owing to high settlement pressure.
Barnacle attachment assays
The barnacle life cycle progresses through six naupliar stages followed by a nonfeeding cyprid larva, which is specialized for settlement. Peak settlement of A. improvisus in the Kiel Fjord usually occurs in July and August (Thomsen et al., 2010; Nasrolahi et al., 2012). During this period, barnacles commonly settle in high numbers within a short period of time. However, settlement pressure may fluctuate drastically between days. Daily observation of settlement panels allowed us to visually estimate settlement pressure during the experiments. We ran a series of field experiments during July–August 2011 as follows:
At the beginning of the cyprid settlement period, panels with monospecies bacterial films were deployed for 7–24 h, depending on settlement pressure, subsequently retrieved, and the number of attached cyprids (irreversible attachment) was counted using a binocular microscope. Five individual experiments were conducted (I–V). Experiment I was carried out before the high settlement pressure period (for 24 h). Experiment II was conducted at the very peak settlement of cyprids (for 24 h). Experiment III was carried out during high settlement pressure but exposure time was reduced to 7 h in order to make it comparable to experiment I in terms of cumulative attachment and in order to examine the role of settlement pressure (comparing experiments II and III) in masking antisettlement effects. Experiment IV was carried out to test the effect of nonviable bacterial films on cyprid attachment (for 7 h). Because several studies have shown the repellent effects of Pseudoalteromonas species on marine invertebrate larvae (e.g. Holmström et al., 2002 and references therein), experiment V was run to test the effect of Pseudoalteromonas species isolated from F. vesiculosus and P. stricta on cyprid attachment. Five to 7 replicates per bacterial film were used for each experiment depending on the number of bacterial strains used in the respective experiment. The single apparatus was fixed on a PVC frame that was deployed at a water depth of 1.5 m. The filmed surfaces served as attachment substratum for cyprids and faced downward to avoid sinking particles from the water column to accumulate on the filters.
Natural assemblage of microorganisms associated with F. vesiculosus
Sampling, experimental design and setup
Thirty F. vesiculosus thalli, each attached to one small rock (defined as one Fucus individual), were collected in the Baltic Sea, Kiel Bight (54°27′N, 10°11′E), at a water depth of approximately 0.5 m in July 2011. Within 2 h of collection, they were brought to the laboratory in individual plastic bags and were transferred to aquaria of the experimental setup in a constant temperature chamber at the IFM-GEOMAR in Kiel. The experimental units (EU) were 20-L plastic aquaria – one for each algal thallus resulting in 30 EUs. Aquaria were filled with FSW from the Kiel Fjord, which was maintained at three different temperature levels: 5, 15 and 20 °C using a water cooler (Titan 2000, Aqua medic) or 300- and 600-W heating rods (SCHEGO, Germany), respectively. All three temperature levels are experienced by F. vesiculosus in its natural habitat during the course of the season. Temperatures above 15 °C enhance biotic stress in Baltic Sea Fucus (Wahl et al., 2011). Thus, the 20 °C treatment can be considered stressful for the brown alga and its biofilm. Light was supplied by neon tubes with an intensity of 100 ± 5 μmol m−2 s−1 and a light/dark regime of 16 : 8. Bubbling stones supplied the tanks with air, and water in the aquaria was exchanged once a week.
Biofilms associated with the surface of F. vesiculosus were harvested after 14 days of incubation at the different temperatures for use in the cyprid settlement assays. One large thallus branch of each individual alga was rinsed with sterile seawater for 5 s to reduce loosely attached particles and was swabbed with three sterile cotton swabs. We know from microscopic analysis that a biofilm on F. vesiculosus is, on average, 11 μm thick (M. Fischer, E. Rickert, unpublished data). To harvest enough biofilm material to obtain a natural concentration of cells in an experimental volume of 1 mL, we swabbed a thallus surface of 900 cm² (= 1 mL/11 μm = 1 cm³/0.0011 cm) and resuspended the microbial cells (after washing, see below) in 1 mL of sterile SW. By removing a natural biofilm from the alga, the original biofilm state is no longer intact. We, therefore, will refer to the suspension of microorganisms as ‘microbial assemblage’ and not as biofilm. Each swab was placed in a 2-mL Eppendorf tube containing 1 mL of sterile seawater, which was vortexed for 15 s to remsove the biofilms from the swab. After discarding the cotton swab, the microbial assemblage was centrifuged for 5 min at 1677 g. The resulting pellet was washed in two steps to remove residual metabolites originating from the host alga. First, the pellet was washed with 1 mL of sterile seawater, the supernatant was discarded, and the centrifugation step was repeated. Again 1 mL of seawater was added. Then, 666 μL of the supernatant was pipetted off. The remaining suspensions of microorganisms from three swabs (each 334 μL) originating from one algal individual were combined (resulting volume = 1002 μL) in the cavity of the attachment apparatus (Fig. 1). The filter was placed on top of the cavity containing the microbial assemblage and sealed tightly with a screw-on lid. This construction allowed water-soluble metabolites produced by the natural assemblage of microorganisms to diffuse through the filter, but retained all organisms < 0.2 μm. The top of the filter served as settlement substratum for the cyprid larvae. The settlement panels were suspended for 16 h in the Kiel Fjord at a water depth of 1.5 m facing downward, after which the number of attached cyprids was counted at ×10 magnification. The assays were repeated in two separate runs owing to limited space on the settlement panels. Both repeats were conducted within 5 days during which experimental conditions were similar. Therefore, we pooled the data of both repeats (resulting in 10 replicates for statistical analysis).
Enumeration of microbial cells
The numbers of microbial cells (bacteria and cells that were not bacteria, that is, mostly diatoms) in the wells of the attachment apparatus at the beginning of the assays were enumerated in a subsample (100 μL) of the suspensions of five replicates per temperature level. The samples were prepared and counted using an epifluorescence microscope following the procedure mentioned previously with the exception that cells in 20 visual fields per sample were counted.
Epibacterial community composition
Sampling
To analyse the epibacterial community composition at the different temperatures via denaturing gradient gel electrophoresis (DGGE), each algal individual was sampled for its associated bacteria. Prior to sampling, approximately 20 cm2 (visual estimation) of algal thallus was rinsed with sterile seawater for 5 s to reduce loosely attached particles. Subsequently, the bacteria were harvested with one sterile cotton swab per sample and stored in 2-ml Eppendorf tubes at −80 °C until DNA extraction.
DNA extraction
DNA was extracted with the QIAamp DNA Mini Kit (Qiagen GmbH) following the manufacturers' protocol for buccal cotton swabs with an additional incubation step at 95 °C for 8 min after the protocol's second lysis step with buffer AL. DNA was eluted with nuclease-free water (Qiagen GmbH) and stored at −20 °C until PCR-DGGE.
PCR-DGGE
The V3 region of 16S rRNA gene sequences of bacteria was amplified using the primer pair 341F-GC (5′-[CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG G] CCT ACG GGA GGC AGC AG-3′) and 534R (5′-ATT ACC GCG GCT GCT GG-3′) (Muyzer et al., 1993) (Eurofins MWG Synthesis GmbH). PCR amplifications were prepared with puReTaq™Ready-To-Go™ Beads (GE Healthcare Europe GmbH), 1 μL of each primer [10 pmol μL−1] and 1 μL of DNA template in a reaction volume of 25 μL per sample. They were performed on a thermocycler (Flex Cycler, Analytik Jena) with following settings: initial denaturation at 94 °C for 2 min, 10 touchdown cycles with initial annealing at 65 °C for 40 s and a reduction of 1 °C per cycle; elongation at 72 °C for 40 s; and denaturation at 94 °C for 30 s. This was followed by 25 cycles of annealing at 55 °C for 40 s, elongation at 72 °C for 40 s and denaturation at 94 °C for 30 s. Final annealing was at 42 °C for 60 s, and final elongation at 72 °C for 5 min. Correct sequence length (233 bp, including the 40 nucleotide GC clamp) of 2.5 μL of PCR amplicons per sample was controlled (100 bp standard ladder, Biozym) via electrophoresis on a 1.5% agarose gel (1× TBE). PCR amplicons were then separated according to GC content on a DGGE gel. A double-gradient gel was cast with a structural gradient of 6–8% acrylamid–bisacrylamid (37.5 : 1) and with a denaturing gradient of 40–80% [100% denaturant is defined as 7 M urea and 40% formamide (v/v)] (Petri & Imhoff, 2001). This gradient was shown to be suitable for the analysis of bacteria associated with F. vesiculosus (Lachnit et al.,2009). Electrophoresis was run in 0.5× TAE buffer at 60 °C and 80 V for 14 h in a C.B.S. Scientific DGGE system. The gel was stained with SYBR Gold (Life Technologies GmbH, Darmstadt, Germany) for 45 min and rinsed with 1× TAE buffer for 30 min prior to gel documentation via UV transilluminator (Vilber).
Statistical analysis
The assumptions of normality and homogeneity of variances were tested with Shapiro–Wilks W test and Levene'e test, respectively. Non-normally distributed data were square-root-transformed. The settlement assays were analysed by one-way anova using the statistica 8 software package (StatSoft). Post hoc comparisons were made with Tukey's HSD.
Bands of the DGGE fingerprint were called with the bare eye, and a Bray–Curtis similarity matrix was constructed based on the presence or absence of bands using the primer 6 software package. The matrix served as basis for analysis of similarity (anosim), and data were visualized by cluster analysis and nonmetric multidimensional scaling. R values generated by anosim indicate the degree of separation between groups and usually lie between 0 and 1 (large values of R, close to 1, indicate complete separation of groups; small values close to 0 imply that they are not or barely separated. Mid-range values of R (0.5) indicate that groups are distinguishable but overlap to a certain degree) (Clarke & Warwick, 2001).
Results
Larval attachment assays with monospecies bacterial films
In general, a higher proportion of A. improvisus cyprids settled on the unfilmed surfaces than on the surfaces with a monospecies bacterial film.
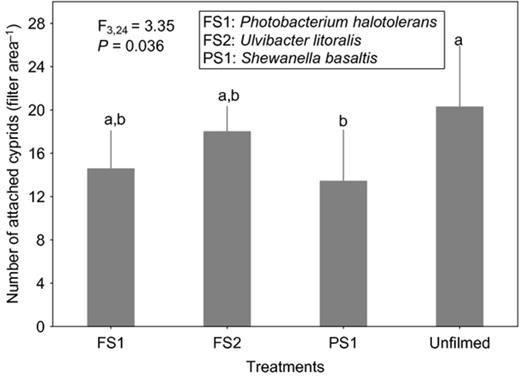
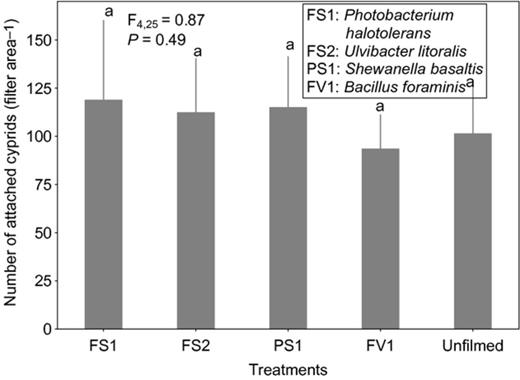
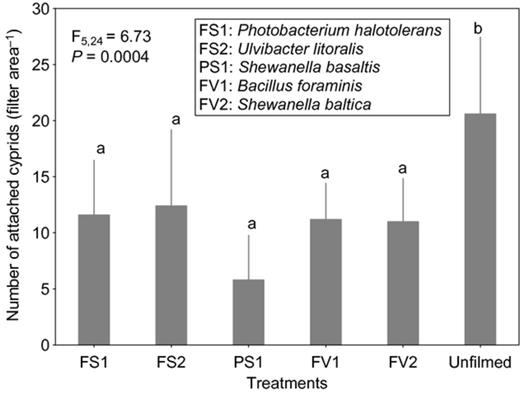
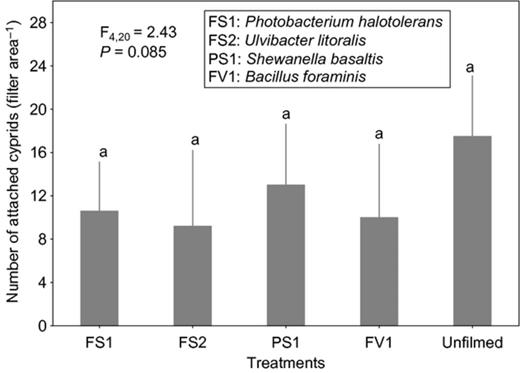
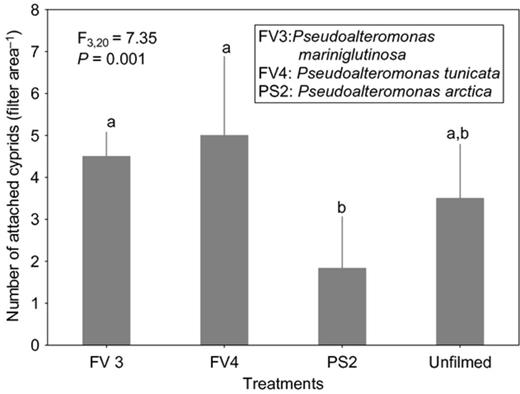
Cyprid attachment on films of the strain affiliated to S. basaltis significantly differed from the attachment on the unfilmed surfaces (Fig. 2). Although the effects of P. halotolerans and U. litoralis films on cyprid attachment were not significantly different from the attachment on the unfilmed surfaces, cyprids tended to attach less to the filmed surfaces than to nonfilmed surfaces (Fig. 2). Under high settlement pressure, the initially observed inhibitive effects of bacterial films on cyprid attachment of all bacterial films disappeared (Fig. 3). When exposure time was reduced (at high settlement pressure), cyprid attachment on all filmed surfaces differed significantly from the unfilmed surfaces (Fig. 4). The repellent effect of S. basaltis tended to be strongest (Fig. 4). No significant difference of cyprid attachment between filmed and unfilmed surfaces was seen after bacterial films were killed (Fig. 5). During the experiments with Pseudoalteromonas, cyprid attachment in the field was low, and on average, only four cyprids attached after 24 h (Fig. 6). Cyprid attachment on P. mariniglutinosa and P. tunicata films did not differ in comparison with the unfilmed surfaces. These two films even showed a slight but nonsignificant inductive effect on cyprid attachment (Fig. 6). In contrast, P. arctica repelled cyprids (Fig. 6).
Experiment I. Effect of monospecies bacterial films isolated from Fucus serratus (FS) and Polysiphonia stricta (PS) on cyprid attachment of Amphibalanus improvisus: mean numbers of cyprids attached after 24 h of exposure in the Kiel Fjord. The unfilmed surface was a filter substrate without bacterial film. Each bar represents the mean of seven replicates. Error bars represent 95% CI. Significant differences at α = 0.05 in Tukey's test are indicated by different letters above the bars.
Experiment II. Effect of monospecies bacterial films isolated from Fucus vesiculosus (FV), Fucus serratus (FS) and Polysiphonia stricta (PS) on cyprid attachment of Amphibalanus improvisus at the very peak settlement: mean numbers of cyprids attached after 24 h of exposure in the Kiel Fjord. The unfilmed surface was a filter substrate without bacterial film. Each bar represents the mean of six replicates. Error bars represent 95% CI. Significant differences α = 0.05 in Tukey's test are indicated by different letters above the bars.
Experiment III. Effect of monospecies bacterial films isolated from Fucus vesiculosus (FV), Fucus serratus (FS) and Polysiphonia stricta (PS) on cyprid attachment of Amphibalanus improvisus: mean numbers of cyprids attached after 7 h of exposure in the Kiel Fjord. The unfilmed surface was a filter substrate without bacterial film. Each bar represents the mean of five replicates. Error bars represent 95% CI. Significant differences at α = 0.05 in Tukey's test are indicated by different letters above the bars.
Experiment IV. Effect of nonviable monospecies bacterial films isolated from Fucus vesiculosus (FV), Fucus serratus (FS) and Polysiphonia stricta (PS) on cyprid attachment of Amphibalanus improvisus: mean numbers of cyprids attached after 7 h of exposure in the Kiel Fjord. The unfilmed surface was a filter substrate without bacterial film. Each bar represents the mean of six replicates. Error bars represent 95% CI. Significant differences at α = 0.05 in Tukey's test are indicated by different letters above the bars.
Experiment V. Effect of Pseudoalteromonas species isolated from Fucus vesiculosus (FV) and Polysiphonia stricta (PS) on cyprid attachment of Amphibalanus improvisus: mean numbers of cyprids attached after 24 h of exposure in the Kiel Fjord. The unfilmed surface was a filter substrate without bacterial film. Each bar represents the mean of five replicates. Error bars represent 95% CI. Significant differences at α = 0.05 in Tukey's test are indicated by different letters above the bars.
Epifluorescence microscopy revealed that the density of bacteria on filters ranged from 1.26 × 106 to 19.3 × 106 cells per filter area (Table 1). There was no significant correlation between the density of bacteria and the number of attached cyprids (Table 1).
Density of attached bacteria and the number of attached cyprids on the filter area of the apparatus
| Bacteria | Mean density of bacteria × 106 cells per filter area | Mean number of cyprid attachment |
| PS1 | 7.699154 | 13.42 |
| FS1 | 3.508008 | 12.5 |
| FS2 | 7.963794 | 18 |
| PS1 | 6.499046 | 5.8 |
| FS1 | 12.40112 | 11.6 |
| FS2 | 16.30916 | 12.4 |
| FV1 | 2.289437 | 11.2 |
| FV2 | 13.2935 | 11 |
| PS1 | 19.29404 | 10 |
| FS1 | 1.261652 | 8.6 |
| FS2 | 12.5919 | 8 |
| FV1 | 8.413065 | 7.5 |
| Bacteria | Mean density of bacteria × 106 cells per filter area | Mean number of cyprid attachment |
| PS1 | 7.699154 | 13.42 |
| FS1 | 3.508008 | 12.5 |
| FS2 | 7.963794 | 18 |
| PS1 | 6.499046 | 5.8 |
| FS1 | 12.40112 | 11.6 |
| FS2 | 16.30916 | 12.4 |
| FV1 | 2.289437 | 11.2 |
| FV2 | 13.2935 | 11 |
| PS1 | 19.29404 | 10 |
| FS1 | 1.261652 | 8.6 |
| FS2 | 12.5919 | 8 |
| FV1 | 8.413065 | 7.5 |
Data on attached bacterial density and number of attached cyprids from experiment I, III and IV in the monospecies bioassay. PS1, Shewanella basaltis; FS1, Photobacterium halotolerans; FS2, Ulvibacter litoralis; FV1, Bacillus foraminis; FV2, Shewanella baltica. The mean density of bacteria was based on counts of five fields at ×1000 magnification under an epifluorescence microscope. There is no correlation between bacterial density and the amount of larval attachment (r2 = 0.007, P = 0.417).
Density of attached bacteria and the number of attached cyprids on the filter area of the apparatus
| Bacteria | Mean density of bacteria × 106 cells per filter area | Mean number of cyprid attachment |
| PS1 | 7.699154 | 13.42 |
| FS1 | 3.508008 | 12.5 |
| FS2 | 7.963794 | 18 |
| PS1 | 6.499046 | 5.8 |
| FS1 | 12.40112 | 11.6 |
| FS2 | 16.30916 | 12.4 |
| FV1 | 2.289437 | 11.2 |
| FV2 | 13.2935 | 11 |
| PS1 | 19.29404 | 10 |
| FS1 | 1.261652 | 8.6 |
| FS2 | 12.5919 | 8 |
| FV1 | 8.413065 | 7.5 |
| Bacteria | Mean density of bacteria × 106 cells per filter area | Mean number of cyprid attachment |
| PS1 | 7.699154 | 13.42 |
| FS1 | 3.508008 | 12.5 |
| FS2 | 7.963794 | 18 |
| PS1 | 6.499046 | 5.8 |
| FS1 | 12.40112 | 11.6 |
| FS2 | 16.30916 | 12.4 |
| FV1 | 2.289437 | 11.2 |
| FV2 | 13.2935 | 11 |
| PS1 | 19.29404 | 10 |
| FS1 | 1.261652 | 8.6 |
| FS2 | 12.5919 | 8 |
| FV1 | 8.413065 | 7.5 |
Data on attached bacterial density and number of attached cyprids from experiment I, III and IV in the monospecies bioassay. PS1, Shewanella basaltis; FS1, Photobacterium halotolerans; FS2, Ulvibacter litoralis; FV1, Bacillus foraminis; FV2, Shewanella baltica. The mean density of bacteria was based on counts of five fields at ×1000 magnification under an epifluorescence microscope. There is no correlation between bacterial density and the amount of larval attachment (r2 = 0.007, P = 0.417).
Larval attachment assays with microbial assemblages
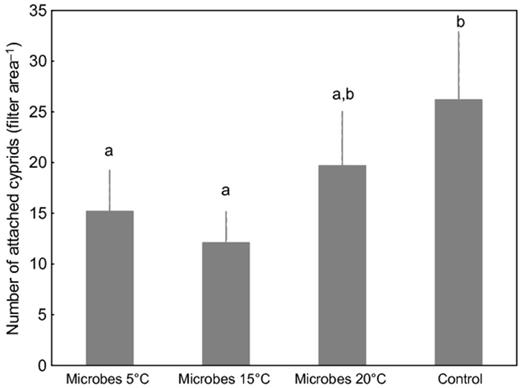
The cyprid attachment assays revealed that on average, 24–54% fewer cyprids attached to substrata with differently treated microbial assemblages than to the filters without microorganisms underneath (controls). Lowest numbers of attached cyprids were found on substrata with microbial assemblages developed at 5 and 15 °C, an effect that was significant relative to control substrata but not relative to the substrata with microbial assemblages developed at 20 °C (Fig. 7). The 20 °C microbial assemblages showed a repellent trend, but this result was not significantly different from the control.
Mean number of attached Amphibalanus improvisus cyprids on apparatus with polycarbonate filters during exposure in the Kiel Fjord for 16 h. Biofilms were detached from their macroalgal host, Fucus vesiculosus, which had been cultured at different temperatures (5, 15, and 25 °C). Filters without the harvested microbial assemblage served as a control substrate. Each bar represents the mean of 10 replicates. Error bars represent 95% CI. Significant differences at α = 0.05 in Tukey's test are indicated by different letters above the bars.
Epibacterial community composition
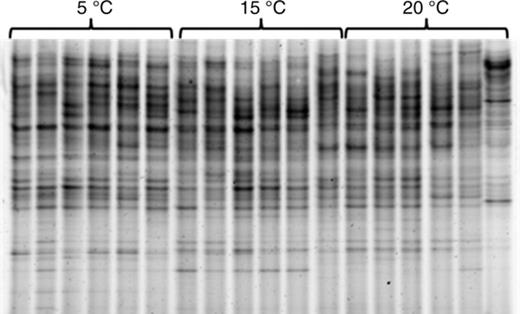
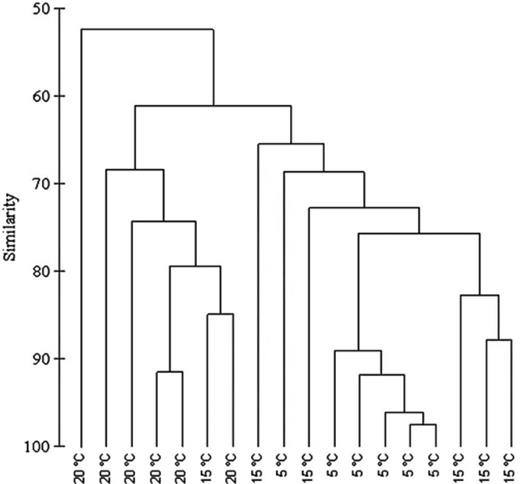
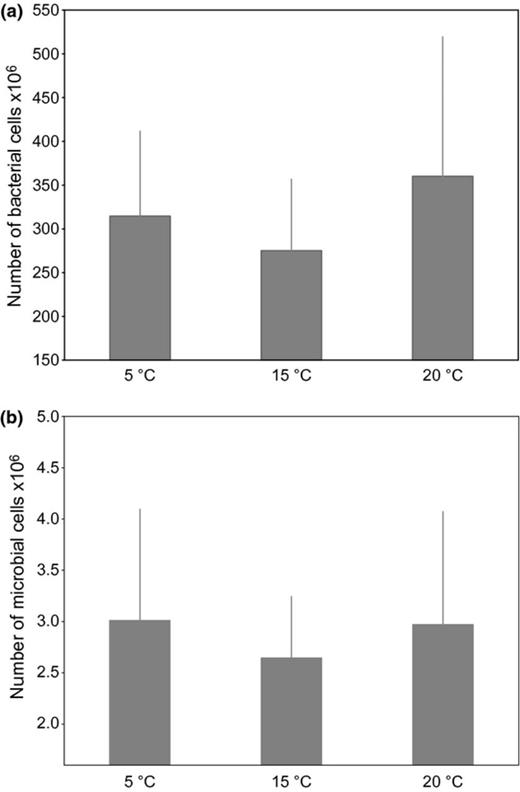
Analysis of the DGGE banding pattern (Fig. 8) of 16S rRNA gene sequences of bacteria sampled from their host, F. vesiculosus, showed that the different temperature treatments led to significant shifts in the epibacterial community composition (anosim global R = 0.524, P = 0.001). The bacterial communities from the different temperature treatments formed clusters with different degrees of similarity (Fig. 9, Fig. S1), as shown by the results of the pairwise permutation tests (Table 2). The bacterial communities of 5 and 20 °C differed as indicated by a high value of R (0.756) (Table 2). The bacterial communities of 5 and 15 °C differed slightly less (R = 0.501), whereas communities from the 15 and 20 °C treatments largely overlapped (R = 0.256). The number of bacterial cells in the cavities of the filter apparatuses during the field attachment assays did not significantly differ between the temperature treatments and was on average 270–360 × 106 cells per cavity. The number of other microorganisms (mostly diatoms) was on average approximately 100-fold smaller and also did not significantly differ between treatments (Fig. 10a and b).
DGGE fingerprint of 16S rRNA gene sequences of epibacteria from Fucus vesiculosus cultured at different temperatures (5, 15 and 20 °C; n = 6 per temperature level).
Dendrogram based on DGGE epibacterial community fingerprint showing similarities in% (group-average linking of Bray–Curtis similarities).
Analysis of similarity (anosim) pairwise tests results of epibacterial community samples (n = 6 per temperature level)
| Groups | R statistic | Significance level | Possible permutations | Actual permutations | # Observed permuted statistics ≥ R |
| 5 °C, 15 °C | 0.501 | 0.002 | 462 | 462 | 1 |
| 5 °C, 20 °C | 0.756 | 0.002 | 462 | 462 | 1 |
| 15 °C, 20 °C | 0.256 | 0.024 | 462 | 462 | 11 |
| Groups | R statistic | Significance level | Possible permutations | Actual permutations | # Observed permuted statistics ≥ R |
| 5 °C, 15 °C | 0.501 | 0.002 | 462 | 462 | 1 |
| 5 °C, 20 °C | 0.756 | 0.002 | 462 | 462 | 1 |
| 15 °C, 20 °C | 0.256 | 0.024 | 462 | 462 | 11 |
Analysis of similarity (anosim) pairwise tests results of epibacterial community samples (n = 6 per temperature level)
| Groups | R statistic | Significance level | Possible permutations | Actual permutations | # Observed permuted statistics ≥ R |
| 5 °C, 15 °C | 0.501 | 0.002 | 462 | 462 | 1 |
| 5 °C, 20 °C | 0.756 | 0.002 | 462 | 462 | 1 |
| 15 °C, 20 °C | 0.256 | 0.024 | 462 | 462 | 11 |
| Groups | R statistic | Significance level | Possible permutations | Actual permutations | # Observed permuted statistics ≥ R |
| 5 °C, 15 °C | 0.501 | 0.002 | 462 | 462 | 1 |
| 5 °C, 20 °C | 0.756 | 0.002 | 462 | 462 | 1 |
| 15 °C, 20 °C | 0.256 | 0.024 | 462 | 462 | 11 |
(a) Mean number of bacterial cells and (b) mean number of other microbial cells (mostly diatoms) used in the field attachment assays per temperature level. Cells were enumerated by epifluorescence microscopy. Each bar represents the mean of five replicates per temperature level. Error bars represent 95% CI; N.S.
Discussion
Knowing that bacteria are key components of the biofilms, especially with regard to their inhibitory effects on macrofoulers (e.g. Khandeparker et al., 2002, 2003; Dobretsov & Qian, 2006), we used bacterial strains isolated from macroalgal surfaces and tested their effects on larval attachment of A. improvisus in field experiments. As only about 5% of bacteria from the natural environment are culturable (Eilers et al., 2000) and, therefore, the results of experiments with bacterial isolates may not reflect interactions in the field, we also used natural microbial assemblages that were harvested from their host F. vesiculosus grown under different temperature conditions.
Marine bacteria associated with basibionts have the potential to protect hosts from micro- and macrofouling (Holmström et al., 1992; Holmström & Kjelleberg, 1999; Steinberg et al., 2002; Dobretsov & Qian, 2004). Our results showed that there were inhibitory effects of monospecies bacterial films on cyprid attachment of A. improvisus. Six isolates from the surfaces of macroalgae tested in this study were inhibitive and two were noninhibitive. O'Connor & Richardson (1996) developed laboratory and field assays with the same barnacle species used in our study but with different monospecies bacterial films (Cobetia marina formerly known as Deleya marina, Alteromonas macleodii and Pseudomonas fluorescens). The authors also found repellent effects on barnacle attachment for all tested strains, although this was substratum-dependent. It seems that several different strains can repel A. improvisus as shown by the study mentioned previously and supported by our results.
In the present study, S. basaltis and P. arctica, isolated from a biofilm associated with the red alga P. stricta, were the most potent inhibitors among all tested isolates. They reduced the number of attached cyprids by 35–67%. Shewanella baltica and B. foraminis isolated from F. vesiculosus had inhibitory effects (~ 48%) similar to the effects of bacterial strains isolated from F. serratus (P. halotolerans and U. litoralis). Pseudoalteromonas tunicata and P. mariniglutinosa isolated from F. vesiculosus did not have any repellent effects on cyprid attachment. Pseudoalteromonas tunicata's neutral effect on attachment of cyprids is surprising because many studies have reported inhibitive effects of this species against settlement of algal spores, invertebrate larvae, bacteria and fungi (e.g. Holmström et al., 2002 and references therein). However, inductive effects of some other Pseudoalteromonas species on marine larval invertebrates have been shown. For instance, Pseudoalteromonas luteoviolacea was identified to be one of the strongest settlement inducers for an Australian echinoid, Heliocidaris erythrogramma (Hugett et al., 2006). Some bacteria express larval-specific activity, that is, inhibit settlement of one species and induce settlement of another species (Maki et al., 1989, 1992; Lau et al., 2003). Different species of barnacle cyprids may also respond differently to similar or even identical cues (e.g. Johnson & Strathmann, 1989; Keough & Raimondi, 1996). In our study, P. tunicata did not have any repellent effect on A. improvisus larvae, whereas this isolate strongly repelled A. amphitrite larvae in the study conducted by Holmström et al. (2002). This target organism specificity can be one explanation for the discrepancy of results from different studies.
Our results show that reduction in barnacle attachment by bacterial films was not density-dependent. These observations coincide with findings of Kavouras & Maki (2004) on the inhibitive effect of monospecies biofilms on zebra mussel larvae. Results of our study are, however, in disagreement with those of Lau et al. (2003), who found a negative correlation between bacterial density and the percent settlement of cyprids. It seems that a certain cell density evokes a repellent effect, and above this density, there is no additional activity. There may have been slight changes in the microbial cell densities and also in the bacterial community composition during the course of the assays that we could not measure. We experienced difficulties in sampling the microbial assemblages at the end of the assays without cell loss, and microscopic enumeration of cells densities was not possible owing to particles and barnacles attached to the filters. However, the assays were of relatively short duration, so we assume that the changes are negligible.
We used the procedure of Lau et al. (2003) in our study and tested cellular viability as a parameter for the settlement inhibition activity of bacterial films. Lau et al. (2003) found that cyprid settlement of A. amphitrite showed no difference on live and nonviable bacterial films. Another study by Lau & Qian (2000) showed that inductive effects of Rhodovulum sp. (Alphaproteobacteria) on larval settlement of the marine polychaete, Hydroides elegans, were strictly dependent on bacterial viability. In our study, inhibitive effects of nonviable (i.e. no metabolic activity) bacterial films on cyprid attachment were weaker than those of live bacterial films. However, all isolates still exhibited inhibitive trends on larval attachment compared to the unfilmed surfaces, suggesting that the inhibitive effect was mediated by both bacterial metabolic activity and direct larval contact with the bacterial film surface (physical existence of bacterial cells).
In our study, inhibitive effects of bacterial films were not observed when cyprid settlement pressure in the field was high. In contrast to our results, O'Connor & Richardson (1998) reported that settlement of A. amphitrite on bacterial films was independent of the number of cyprids they used in their assays in the laboratory. During surface exploration, barnacle cyprids use a proteinaceous secretion to attach temporarily and walk across surfaces, leaving behind a so-called footprint. This secretion also functions as settlement cue for subsequently exploring larvae (Dreanno et al., 2006a, b). Barnacles often settle gregariously (Crisp, 1974), which could explain the high settlement rates of cyprids when large numbers of cyprids are present in the field. In laboratory experiments, however, cyprid density is usually lower than that in natural habitats during peak settlement. Consequently, the magnitude of settlement observed in laboratory experiments may not be ecologically representative (Gotelli, 1990; Clare et al., 1994).
During peak settlement of barnacles in the Kiel Bight, we have observed that F. vesiculosus thalli can become heavily fouled by A. improvisus (Rohde et al., 2008; Fig. S2). This observation indicates that results from the monospecies bacterial films, that is, no repelling effect on cyprids when settlement pressure was severe, reflect realistic scenarios in the field. The thallus surface is essential for light and nutrient uptake (Wahl, 2008). Severe overgrowth by macrofoulers can, therefore, be disadvantageous for the host alga because photosynthesis is reduced and thus growth (Rohde et al., 2008). Obviously, F. vesiculosus is not covered by a monospecies biofilm in the field but by a complex bacterial community (Lachnit et al., 2011). Therefore, in addition to the monospecies films, we tested the effect of natural epibiotic microbial assemblages on cyprid attachment, which closer resemble the natural biofilm state than monospecies films do. We could not conduct the attachment assays with biofilms that were identical with those on the alga, because we removed the biofilm from its host, possibly losing biofilm members in the cotton swabs. We followed this procedure to exclude or at least minimize the potential direct effects of the host alga on barnacle attachment. Despite this limitation, the microbial assemblages had repellent effects on barnacle larvae, most pronounced with the microorganisms from algae treated at 5 and 15 °C. The repellent effect of bacterial biofilms on invertebrate larval attachment can be explained by bacterial abundance (e.g. Dobretsov & Qian, 2006) and by the bacterial community composition (e.g. Qian et al., 2003), both of which were analysed in our study. Neither the number of bacterial cells nor the number of other microorganisms (mostly diatoms) differed between the biofilms from different temperatures. Apparently, the number of microbial cells was not responsible for the strongest repellent effects of the 5 and 15 °C microbial assemblages. These results coincide with previous studies on the attachment of two barnacle species that were not affected by the abundance of microorganisms (Qian et al., 2003; Thiyagarajan et al., 2006). Our results can rather be explained by the bacterial community composition, which differed most between 5 and 20 °C and least between 15 and the 20 °C, respectively. The 15 °C microbial assemblages repelled on average about 39% more and the 5 °C microorganisms on average 23% more barnacle larvae than the 20 °C microbial assemblages did. We would, therefore, have expected the microbial communities from the lower (5 and 15 °C) and high (20 °C) temperature treatments to form distinct communities responsible for the observed effects on cyprid attachment. However, the picture was not as clear as expected: the 15 and 20 °C microbial communities did not form distinct clusters but largely overlapped in their community composition. In a previous study, temperature had a direct effect on the bacterial community composition on artificial substrate, which in turn affected the attractiveness of the biofilm to barnacle cyprids (Lau et al., 2005). In their study, the bacterial communities differed more among each other than the ones in our study did. Our results indicate that not large but small shifts in the community composition (which in our study resulted from a temperature shift of 5 °C) or in the relative abundance of single community members may have accounted for a stronger repellent effect of the microbial assemblage. It remains to be tested which members of the bacterial community are responsible for the repellent effects. Candidates could be the single strains that showed repellent effects in the study at hand. However, it remains unclear whether they would play the same role in a complex community. We cannot rule out that other members of the microbial assemblage (e.g. diatoms) or metabolites from the host algae may have caused or at least contributed to the repulsion of barnacles. Fucus vesiculosus has been shown to produce secondary metabolites (phlorotannins) with the potential to directly repel cyprids (Brock et al., 2007). However, the microbial assemblages used in our study were thoroughly washed to remove residual host algal water-soluble phlorotannins and other water-soluble compounds. Microalgal cells (mostly diatoms) were present in the tested microbial community. They were not identified further, and therefore, we do not know whether bioactive species were present. In any case, diatom cells were outnumbered by bacteria by far (100-fold). Considering the repellent effect of several monospecies biofilms in this study and the undisputed key role of bacteria in larval settlement (reviewed in Hadfield, 2011), it is likely that the bacteria in the natural microbial assemblages were at least in part responsible for the repellent effects against barnacle larvae. There is evidence that bacteria produce water-soluble compounds that play an inhibitive role in the attachment of the polychaete H. elegans (Dobretsov & Qian, 2002). To our knowledge, in most studies (e.g. Qian et al., 2003; Lau et al., 2005) on the inhibitive effects of complex biofilms on barnacle attachment, assays were conducted with larvae being in direct contact with the biofilm, thus testing surface bound and water-soluble compounds simultaneously. In our study, the attachment apparatus was constructed in a way that only allowed water-soluble compounds from the natural microbial assemblage to diffuse through the filters. The observed repellent effect must, therefore, have been caused by one or several water-soluble compounds emitted by the bacteria (and possibly diatoms) rather than by surface bound compounds of the extracellular polymeric substances. The nature of the compounds was not analysed and their identification will need to be addressed in future studies.
Many antibacterial compounds have been extracted from algae (reviewed in Goecke et al., 2010), indicating that many algae species actively defend their surface from epibiosis (for examples see Harder, 2008). Some algal species have been shown to regulate their surface-associated bacteria to their own benefit (Delisea pulchra: Maximilien et al., 1998; Bonnemaisonia asparagoides: Nylund et al., 2010; Dictyosphaeria ocellata: Sneed & Pohnert, 2011). This regulatory capacity has also been suggested for F. vesiculosus (Lachnit et al., 2010). In our study, the microbial assemblages from algae treated at the lower temperatures (5 and 15 °C) were the most repellent. Therefore, it seems possible that the alga is in a healthy state at lower temperatures, allowing it to maintain a beneficial epibacterial community, and that the alga's chemical control of the biofilm could be impaired at higher temperatures causing stress. Although this idea is speculative, we find it deserves attention in future studies investigating effects of climate change. In the course of climate change, water surface temperatures in the Baltic Sea could increase by up to 4 °C by 2100 (BACC, 2008). This rise in temperature could indirectly reduce the general performance of F. vesiculosus via weakening of the protective biofilm, leading to higher fouling of its surface and consequently reduced available solar energy. Besides this, higher water temperatures during the summer months coincide with high settlement pressure of barnacles as well as increased grazing pressure, and therefore, can generate synergistic stressful interactions (Wahl et al., 2011).
We conclude that the complex biofilm associated with macroalgae can protect its host from unwanted colonizers. This indirect defence mechanism may strengthen the direct antifouling defence of the macroalga against macrofoulers. As indicated by the results of the monospecies films, this protective effect may not withstand severe fouling pressure but may minimize biotic stress for the algae caused by fouling of its surface at times when fouling pressure is moderate. Temperature stress may also reduce the protective effect of the alga's biofilm. Thus, an increase in water temperature owing to global warming may result in multiple stressors reducing the performance of the macroalga.
Authors' contribution
A.N. and S.B.S. contributed equally to this work.
Acknowledgements
We are grateful to Mahasweta Saha and Hajar Zarei for their help with bacterial cultures and laboratory work and acknowledge Frauke Symanowski and Katrin Kleinschmidt for isolation of bacteria and help with identification. We thank Ralf Schwarz and his colleagues at the TLZ for constructing the attachment apparatuses. We thank two anonymous reviewers for their valuable comments and suggestions that improved this manuscript substantially. A.N. gratefully thanks the Ministry of Science, Research and Technology (MSRT) of Iran for awarding him a scholarship to pursue a PhD. This research was supported by the German Research Council (DFG, project number D1238).
References
Supporting Information
Additional Supporting Information may be found in the online version of the article:
Fig. S1. Non-metric multidimensional scaling (n-MDS) plot visualizing differences and overlaps between epibacterial community compositions.
Fig. S2. Fucus vesiculosus collected in the Kiel Fjord with severe fouling by barnacles.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Author notes
Editor: Angela Sessitsch