-
PDF
- Split View
-
Views
-
Cite
Cite
Maria V. Orlova, Sergey V. Tarlachkov, Galina A. Dubinina, Elena V. Belousova, Maria N. Tutukina, Margarita Y. Grabovich, Genomic insights into metabolic versatility of a lithotrophic sulfur-oxidizing diazotrophic Alphaproteobacterium Azospirillum thiophilum, FEMS Microbiology Ecology, Volume 92, Issue 12, December 2016, fiw199, https://doi.org/10.1093/femsec/fiw199
Close - Share Icon Share
Diazotrophic Alphaproteobacteria of the genus Azospirillum are usually organotrophs, although some strains of Azospirillum lipoferum are capable of hydrogen-dependent autotrophic growth. Azospirillum thiophilum strain was isolated from a mineral sulfide spring, a biotope highly unusual for azospirilla. Here, the metabolic pathways utilized by A. thiophilum were revealed based on comprehensive analysis of its genomic organization, together with physiological and biochemical approaches. The A. thiophilum genome contained all the genes encoding the enzymes of carbon metabolism via glycolysis, tricarboxylic acid cycle and glyoxylate cycle. Genes for a complete set of enzymes responsible for autotrophic growth, with an active Calvin–Benson–Bassham cycle, were also revealed, and activity of the key enzymes was determined. Microaerobic chemolithoautotrophic growth of A. thiophilum was detected in the presence of thiosulfate and molecular hydrogen, being in line with the discovery of the genes encoding the two enzymes involved in dissimilatory thiosulfate oxidation, the Sox-complex and thiosulfate dehydrogenase and Ni–Fe hydrogenases. Azospirillum thiophilum utilizes methanol and formate, producing CO2 that can further be metabolized via the Calvin cycle. Finally, it is capable of anaerobic respiration, using tetrathionate as a terminal electron acceptor. Such metabolic versatility is of great importance for adaptation of A. thiophilum to constantly changing physicochemical environment.
INTRODUCTION
Diazotrophic Alphaproteobacteria of the genus Azospirillum are widespread in a variety of ecosystems, mainly in soils and in the root system of plants from various geographical zones (Bashan, Holguin and de-Bashan 2004). Among the known species, only two were isolated from aquatic ecosystems. Azospirillum largimobile was found in deep groundwater samples (Dekhil et al.1997). A recently described species A. thiophilum inhabits a deep mineral sulfide spring with unstable physicochemical conditions, which is an unusual habitat for diazotrophs (Lavrinenko et al.2010). Unlike soil ecosystems, it contains very low amounts of organic matter. The capability of this diazotrophic organism to grow lithoautotrophically using reduced sulfur compounds, abundant in its habitat, as electron donors is therefore of interest.
We have previously reported chemolithoheterotrophic growth of A. thiophilum under microaerobic conditions in the presence of thiosulfate (Frolov et al.2013). The cells were found to contain two enzyme systems involved in thiosulfate oxidation, thiosulfate dehydrogenase, TsdA, which catalyzes incomplete oxidation of thiosulfate to tetrathionate, and a thiosulfate-oxidizing Sox-complex responsible for complete thiosulfate oxidation to sulfate. Activities of these enzymes were determined, and expression of the soxB gene was shown under microaerobic growth conditions, in contrast to aerobic growth. Coupling of thiosulfate oxidation to the electron transfer chain operation was demonstrated (Frolov et al.2013).
Recently, the genomes of some representatives of the Azospirillum genus (e. g. A. lipoferum, A. brasilense, A. halopraeferens, A. humicireducens) were sequenced and deposited in GenBank (Wisniewski-Dye et al.2011; Rivera et al.2014). However, no detailed analysis of the metabolic pathways enabling environmental plasticity of the Azospirillum representatives was undertaken.
The genome of A. thiophilum was sequenced by the two research groups almost simultaneously: we obtained a complete genome sequence and deposited it in GenBank (Fomenkov et al.2016), while another group also deposited the whole-genome shotgun sequence in GenBank (NZ_LAEL00000000.1) (Kwak and Shin 2016). The work of Kwak and Shin confirmed the previously established ability of A. thiophilum to fix dinitrogen and to grow lithotrophically in the presence of thiosulfate using the Sox-system (Lavrinenko et al.2010; Frolov et al.2013). They also identified the genes of A. thiophilum encoding ribulose-1,5-bisphosphate carboxylase and phosphoribulokinase, however, they had not demonstrated capacity of this organism for autotrophic growth (Kwak and Shin 2016).
In the present work, a detailed analysis of the complete genome complemented by physiological and biochemical approaches has been used to reconstruct metabolism of A. thiophilum and to show its capacity for conversion of C1 compounds, thiosulfate, H2 and overall catabolic versatility.
MATERIALS AND METHODS
Cultivation
Azospirillum thiophilum BV-ST was grown in liquid MPSS (modified peptone-succinate-salts) medium containing the following components (g L−1): (NH4)2SO4, 1.0; CaCl2 · 2H2O, 0.03; MgSO4 · 7H2O, 1.0; Na2S2O3 · 5H2O, 1.0; sodium succinate, 1.0; peptone, 2.0 (Caraway and Krieg 1974) and vitamin and trace element solutions (Pfennig and Lippert 1966).
Microaerobic autotrophic cultivation was carried out in the same medium without peptone and sodium succinate, with NaHCO3 (0.5 g L−1) as a sole carbon source and thiosulfate (1 g L−1) or H2 (50% of the gas phase) as sources of electrons. Bacteria were grown in 20 mL of liquid medium dispensed into 100 mL vials with butyl rubber stoppers and metal screw caps. Microaerobic conditions were established by filling the vials with freshly boiled sterile medium to capacity, displacing a known amount of liquid with argon, and adding air to 2% O2 concentration. The gas phase was sterilized by filtering through 0.2-μm membranes (Millipore). The vials were inoculated with late-exponential culture (1 mL). All analyses were carried out after three to four transfers in a relevant medium. In order to avoid a significant decrease in O2 concentration during growth, the ratio of liquid and gas phases was 1 : 4. Cultivation with methanol (0.5 g L−1) or formate (0.5 g L−1) was carried out with or without NaHCO3.
DNA isolation, genome sequencing and annotation
DNA was isolated using the standard phenol/chlorophorm extraction protocol. Sample quality was assessed by electrophoresis in 1% agarose gel in 1× TBE buffer, and DNA concentration was determined using a ND-1000 spectrophotometer (NanoDrop Technologies, USA).
Sequencing and assembling of the genome, as well as the GenBank accession numbers of the chromosomes, were reported previously (Fomenkov et al.2016). Initial genome annotation of A. thiophilum was taken from our previous work. Additional gene prediction and functional annotation analyses were performed using RAST (Aziz et al.2008) and KAAS (Moriya et al.2007) with default parameters. Functions of some genes were checked manually by BLAST (Altschul et al.1990) using the non-redundant protein database. Pairwise sequence alignments were performed using the Needleman–Wunsch alignment algorithm (http://www.ebi.ac.uk/Tools/psa/emboss_needle/). These data sources were combined together to identify the metabolic pathways and to assert a product description for the genes.
Circular map of the chromosomes was made with DNAPlotter (http://www.ncbi.nlm.nih.gov/pubmed/18990721).
Expression of the rbcL gene
RNA was isolated with ExtractRNA reagent (Evrogen, Russia) according to the manufacturer's protocol. RNA quality was assessed by electrophoresis in 2% agarose gel supplemented with 2.2 M formaldehyde. RNA concentration was measured with the Qubit® RNA HS Assay Kit (Thermo Fisher Scientific, USA) on a Qubit 2.0 fluorimeter (Thermo Fisher Scientific, USA). 1000 ng of RNA was then reverse transcribed using M-MulV (SybEnzyme, Russia) and Eppendorf Mastercycler Personal according to the manufacturer's protocol. Quantitative RT-PCR was performed using SYBR Green I on the Bio-Rad CFX96TM Real-Time System (Bio-Rad, USA). To find the temperature optimal for amplification, a temperature gradient was applied. The resulting program included initial denaturation at 95°C for 3 min; followed by 39 cycles of 20 s denaturation at 95°C, 20 s primer annealing at 58°C, and 30 s of elongation at 72°C. The rbcL gene coding for the ribulose bisphosphate carboxylase large chain was amplified with the primers RbcL_F2 (5′-AACCCAAGGACACCGACATC-3′) and RbcL_R2 (5′-GGTCTTCAGATAGGCGACCG-3′) picked by PrimerBLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/).
Cell lysate preparation
To prepare cell lysates, the culture was centrifuged for 30 min at 5000 g at 4°C to collect cells. Pellets were then washed with 0.1 M Tris-HCl buffer (pH = 7.5) and centrifuged for 20 min. Cell lysates were obtained by ultrasonic treatment for 2 min on an ice bath using a UZDN-2T sonicator (Akadempribor, Ukraine) at 500 W and 22 kHz. The cytoplasmic fraction was obtained in a supernatant after 30 min centrifugation at 9000 g, 4°C.
Protein content was determined by the Lowry method (Lowry et al.1951). Prior to analysis, cells were hydrolised with 1M NaOH at 90°C for 10 min.
Enzymatic activity assays
Dehydrogenase activities, malate dehydrogenase (EC 1.1.1.37; 1.1.1.40) (Peak, Peak and Ting 1972), isocitrate dehydrogenase (EC 1.1.1.42) (Glaeser and Schlegel 1972) and formate dehydrogenase (Hartmann and Leimkuhler 2013), were measured in the supernatant at λ = 340 nm as the rates of oxidation (reduction) of the coenzymes, NADH or NADPH, in the relevant reaction mixtures. Hydratase activities were determined based on differences in absorption of citrate and cis-aconitate for aconitate hydratase (EC 4.2.1.3) (Reyns and Leonis 1974), and malate and fumarate for fumarate hydratase (EC 4.2.1.2) (Rice and Pon 1978) at λ = 240 nm. Activity of isocitrate lyase (EC 4.1.3.1) was determined based on the difference in absorption at λ = 324 nm of the complex resulting from interaction between phenylhydrazine, a component of the reaction mixture, and glyoxylate produced in the enzymatic reaction (Khan et al.1979). Citrate synthase (EC 4.1.3.7) and malate synthase (EC 4.1.3.2) activities were determined at λ = 412 nm based on the rate of emergence of thiol groups of the free CoA as detected with the Ellman's reagent (Schater and Schonheit 1991). Activity of succinate dehydrogenase (EC 1.3.99.1) was measured by the phenazine methosulfate method at λ = 600 nm (Cooper and Beevers 1969).
Phosphoribulokinase activity was measured using the rate of phosphorus accumulation from alkaline hydrolysis of ribulose-1,5-bisphosphate (Hurwitz et al.1956).
Activity of hydrogenase (EC 1.12.1.2) was determined by reduction of oxidized methylviologen (600 nm, 30°C) (Children and Kenneth 1984). Specific activities were expressed in μmol min−1 mg−1 protein.
Activity of carbonic anhydrase was determined by measuring the time of transition of bromothymol indicator color from blue to yellow (Wilbur and Anderson 1948). The activity of the enzyme was calculated in arbitrary units (a.u.) of Wilbur and Andersen.
When both S2O32− and S4O62− ions were present in the medium, they were determined by separate iodometric titration (Reznikov, Mulikovskaya and Sokolov 1970).
RESULTS
Genome characterization
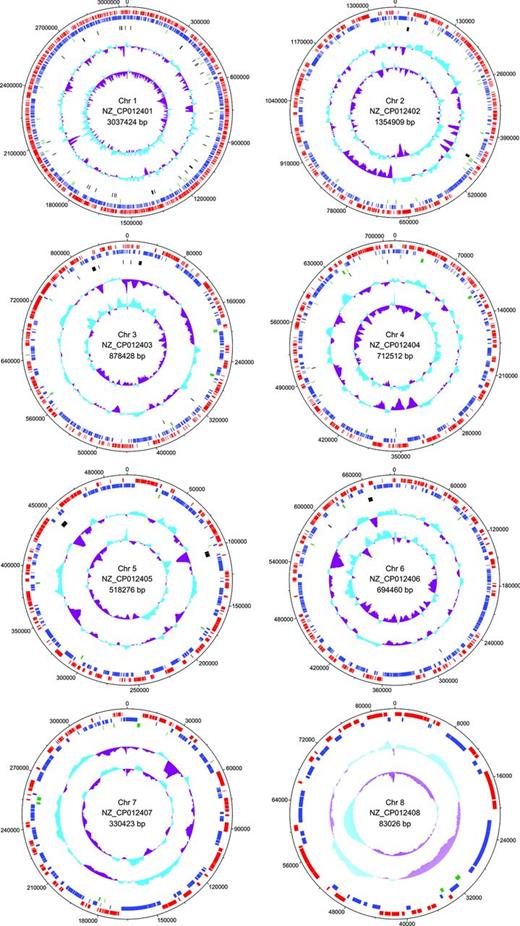
The Azospirillum thiophilum genome contains eight circular chromosomes (Fig. 1) (GenBank accession nos. NZ_CP012401.1–NZ_CP012408.1), with a total length of 7.6 Mbp and an average G+C content of 68.2%. Seven closed circled genetic elements have the same coverage of around 370 indicating that they are represented with equal copy numbers and are probably replicated with approximately equal rate, except for the chromosome 7 that has a double coverage of 660 (Fomenkov et al.2016). A total of 6420 protein-coding genes (CDS), 79 tRNAs, four ncRNA and 26 rRNAs were predicted. 4404 CDS have sequence similarity to known genes and 2016 CDS with unknown functions. Average gene length is approximately 1010 bp and a coding density is 0.84 genes per kb.
Circular map of the A. thiophilum BV-ST chromosomes. Most outer tracks represent predicted ORFs on the forward (red) and reverse (blue) strands. Green track represents pseudogenes, black track represents rRNAs, tRNAs and ncRNAs. The two inner tracks display the GC-plot and the GC-skew.
Autotrophic growth
We have previously shown that A. thiophilum is capable of chemoorganoheterotrophic and chemolithoheterotrophic growth (Frolov et al.2013). Its ability to grow autotrophically remained doubtful. Although Kwak and Shin (2016) found the genes encoding ribulose-1,5-bisphosphate carboxylase and phosphoribulokinase in the A. thiophilum genome, the autotrophic growth of this organism was not demonstrated.
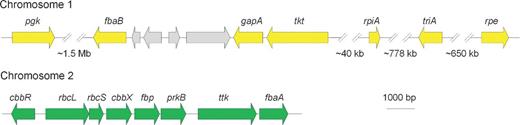
Analysis of the complete genome sequence obtained in our laboratory showed that it contains a complete set of genes encoding the Calvin–Benson–Bassham cycle (Fig. 2; Table S1, Supporting Information).
Organization of the genes coding for the enzymes of the Calvin–Benson–Bassham cycle: pgk - Phosphoglycerate kinase (EC 2.7.2.3); fbaB - Fructose-bisphosphate aldolase class I (EC:4.1.2.13); gapA - NADPH-dependent glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.13)/ NAD-dependent glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.12); tkt - Transketolase (EC 2.2.1.1); rpiA - Ribose 5-phosphate isomerase B (EC 5.3.1.6); rpe - Ribulose-phosphate 3-epimerase (EC:5.1.3.1); triA - Triosephosphate isomerase (EC 5.3.1.1); cbbR - RuBisCO operon transcriptional regulator CbbR; rbcL - Ribulose bisphosphate carboxylase large chain (EC 4.1.1.39); rbcS - Ribulose bisphosphate carboxylase small chain (EC 4.1.1.39); cbbX - Probable RuBisCo-expression protein CbbX; fbp - Fructose-1,6-bisphosphatase, type I (EC 3.1.3.11); prkB - Phosphoribulokinase (EC 2.7.1.19) and fbaA - Fructose-bisphosphate aldolase class II (EC 4.1.2.13).
The gene encoding sedoheptulose-1,7-biphosphatase was not found in A. thiophilum. However, it can be substituted by fructose-1,6-biphosphatase, taking into account its double specificity to sugars in bacteria (Tamoi et al.1996).
Genes encoding the enzymes of the Calvin–Benson–Bassham cycle (pgk, fba, gapA, tktA, rpiA, rpe, tpiA, cbbR, rbcL, rbcS, cbbX, fbp, prkB, and fbaA) do not form a single cluster in the genome, but are rather distributed between three chromosomes (1, 2 and 5). Most of these genes are located in the first and the second chromosomes, and represented with a single copy. NADPH/NADP-dependent glyceraldehyde-3-phosphate dehydrogenase is the first exception being represented with two copies of the gapA gene in the first and fifth chromosomes. The second exception is transketolase with three copies of the tktA gene located in the first, second and fifth chromosomes.
To confirm operation of the Calvin–Benson–Bassham cycle experimentally, we measured the activity of several enzymes participating in CO2 assimilation and reduction under autotrophic growth.
Activity of phosphoribulokinase, one of the key enzymes of the Calvin–Benson–Bassham cycle, was revealed in autotrophically grown A. thiophilum (2.50 ± 0.13 μmol min−1 mg−1 protein), but it was not detected in heterotrophically grown cells. Under lithoautotrophic and lithoheterotrophic growth, activity of carbonic anhydrase, the enzyme involved in conversion of bicarbonate ions to CO2, which directly interacts with Rubisco was 19.60 ± 1.00 and 3.30 ± 0.17 Wilbur and Andersen a.u., respectively.
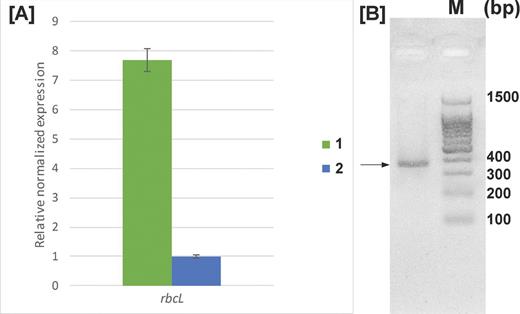
In order to confirm the operation of the Calvin–Benson–Bassham cycle in A. thiophilum, expression of the rbcL gene encoding the Rubisco large subunit was determined for the culture growing under auto- and heterotrophic conditions. qRT-PCR demonstrated that the rbcL-mRNA level under autotrophic conditions was approximately 8-fold higher than that in heterotrophic conditions (Fig. 3A). Homogeneity of the PCR product was confirmed by electrophoresis in 2% agarose gel (Fig. 3B).
(A) Expression of the rbcL gene during (1) autotrophic and (2) heterotrophic growth. Error bars were calculated based on three independent experiments. (B) Amplification of the rbcL gene fragment with the RbcL_F2/RbcL_R2 primer pair (expected product length 353 bp). M - DNA Molecular weight ladder. The product of the expected length (353 bp) is indicated by the arrow.
The genome of A. thiophilum also contains the gene encoding type IV Rubisco. This protein, being structurally similar to Rubisco, is incapable to catalyze the ribulose-1,5-bisphosphate-dependent CO2 fixation (Hanson and Tabita 2003).
Thus, CO2 fixation in autotrophically growing A. thiophillum is carried out via the Calvin–Benson–Bassham cycle.
Lithoautotrophic growth in the presence of thiosulfate
Analysis of the A. thiophilum genome revealed the presence of the soxABCDXYZ gene cluster suggesting a possibility of thiosulfate oxidation by the multienzyme Sox-complex (Fomenkov et al.2016; Kwak and Shin 2016).
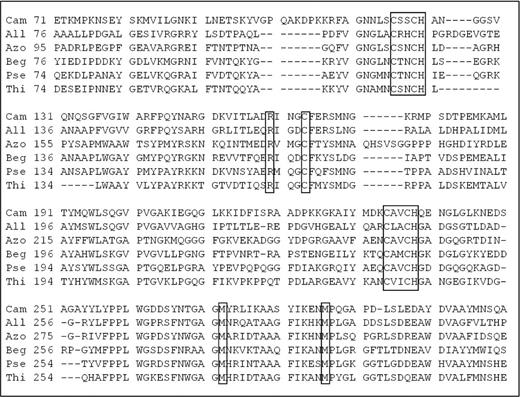
Despite the fact that availability of the tsdA gene encoding TsdA was previously predicted, it was not annotated in the A. thiophilum BV-ST genome. However, a protein belonging to the family of cytochromes was found in the NCBI database, accompanied by the discovery of the gene homologous to tsdA (cyt c) on the third chromosome. Alignment of these sequences with the sequences of homologous proteins from Gammaproteobacteria allowed detecting conservative motifs similar to those in previously described TsdA (Fig. 4).
Multiple sequence alignment of TsdA from Campylobacter jejuni (Cam) and Allochromatium vinosum (All) with homologous amino acid sequences from Beggiatoa sp. PS (EDN69405.1) (Beg), Pseudomonas sp. BAY1663 (EXF46491.1) (Pse), Thiobacillus denitrificans (WP_011310641.1) (Thi) and Azospirillum thiophilum (Azo). Conservative amino acids specific for TsdA are shown in boxes.
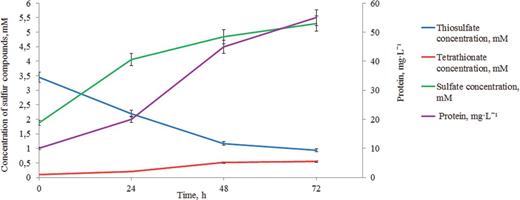
In microaerobic conditions during lithohetero- or lithoautotrophic growth A. thiophilum is capable of dissimilatory thiosulfate oxidation leading to formation of sulfate and tetrathionate. Sulfate is formed due to SOX-system, whereas the TsdA activity results in synthesis of tetrathionate (Fig. 5). Activity of the corresponding enzymatic systems, as well as expression rate of the soxB gene, was measured earlier (Frolov et al.2013). It was also established that the molar amount (per mg of protein) of thiosulfate oxidized by A. thiophilum growing lithoautotrophically in microaerobic conditions was increased 5–6-fold as compared to the mixotrophic growth suggesting an enhanced role of thiosulfate as the electron donor.
Dynamics of thiosulfate oxidation to tetrathionate and sulfate and dynamics of cell biomass accumulation for A. thiophilum during microaerobic growth in the presence of thiosulfate as an electron donor. The left Y-scale represents molar concentrations of thiosulfate, tetrathionate and sulfate. The right Y-scale represents protein concentration, mg L−1.
Importantly, the genes encoding the enzymes involved in dissimilatory oxidation of sulfide to elementary sulfur (sqr), sulfur to sulfite (dsrABEFHCMLJOPNR) and sulfite to sulfate (sor, yed, apr and sat) were not found.
Lithoautotrophic growth in the presence of molecular hydrogen
Azospirillum thiophilum was found to contain the hyaAB genes, which encode the large and small catalytic subunits of the group I membrane-bound [NiFe] hydrogenase (Volbeda et al.1995; Vignais, Billoud and Meyer 2001). This group of extracytoplasmic enzymes employs both hydrogen oxidation and generation of proton-motive force (Vignais, Billoud and Meyer 2001). The hyaCb gene, also found in the analyzed genome, encodes the cytochrome b subunit, which, together with the hydrophobic C-terminal segment of the small subunit attaches hydrogenase to the cytoplasmic membrane (Vignais, Billoud and Meyer 2001; Vignais 2008). Apart from the catalytic subunits, A. thiophilum genome contains the hypABCDE genes. Being the part of the group I hydrogenase cluster, proteins encoded by these genes participate in maturation of [NiFe] hydrogenases (Wolf et al.1998) (Table S1, Supporting Information).
Biochemical analysis was used to reveal hydrogenase activity in A. thiophilum (11.8 ± 0.59 μmol min−1 mg−1 protein). Lithotrophic growth of A. thiophilum in the presence of H2 occurred only under autotrophic microaerobic conditions, when H2 consumption was up to 20% of the introduced H2 (50% of the gas phase).
Methylotrophic growth
Analysis of genome revealed that A. thiophilum utilizes methanol and formate to get some electrons. CO2 formed during this process can further be utilized via the Calvin cycle.
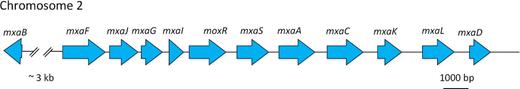
In A. thiophilum the genes encoding the enzymes involved in methanol oxidation to formaldehyde are located in a single cluster on chromosome 2 (Fig. 6; Table S1, Supporting Information).
Organization of the genes coding for the enzymes of the mxa-cluster of classical MDH. mxaB - a response regulator involved in regulation of methanol oxidation; mxaF - methanol dehydrogenase large subunit protein; mxaJ - MxaJ, protein involved in methanol oxidation; mxaG - Cytochrome c-L precursor); mxaI - methanol dehydrogenase, small subunit (EC 1.1.99.8); mxaR - methanol dehydrogenase regulatory protein MoxR; mxaS - hypothetical protein MxaS; mxaA - MxaA protein; mxaC - MxaC, protein involved in Ca2+ insertion into methanol dehydrogenase; mxaK - MxaK protein; mxaL - MxaL protein; mxaD - MxaD protein.
Three types of methanol dehydrogenases are presently known. Methanol dehydrogenases (MDH) of the first type are enzymes consisting of subunits of different types: catalytic (the mxaFI genes), cytochrome c electron acceptors (the mxaG gene), subunits required for the transport of calcium ions to the active center (the mxaACKL genes) and subunits with an unknown function (the mxaRS genes). The second type of methanol dehydrogenases (MDH2) is found in members of the orders Burchorderiales and Rhodocyclales. This enzyme consists of a single type of subunits. The recently described third type of methanol dehydrogenases (XoxF protein) is as yet insufficiently characterized (Chistoserdova 2011).
Two types of MDH were found in A. thiophilum genome, the MxaF methanol dehydrogenase of the first type (77.6% identity to the homologous protein of Methylobacterium extorquens DM4 (CAX26759.1)) and the XoxF methanol dehydrogenase of the third type (64.3% identity to the homologous protein of M. extorquens DM4 (CAX24146.1)).
The genome of A. thiophilum also contained the genes of the mxa cluster coding for the classical MDH. Aligning of amino acid sequences of A. thiophilum proteins encoded by these genes with amino acid sequences of M. extorquens, the first bacterium, in which the first type MDH was described, was carried out. The identity between A. thiophilum and M. extorquens MxaI, MxaA, MxaC, MxaD, MxaK, MxaL, MxaJ, MxaG, MxaS, MxaR and MxaB sequences varied from 41.3 to 68.8% (Table S2, Supporting Information). Together they formed the canonical type of the mxa cluster (Lidstrom et al.1994; Anthony 2004; Lidstrom 2006).
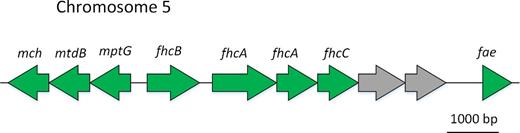
Formaldehyde oxidation to formate occurs in A. thophilum via the tetrahydromethanopterine pathway. The genome was found to contain the genes encoding all enzymes of this pathway (Fig. 7; Table S1, Supporting Information), located in the fifth chromosome.
Organization of the genes coding for the enzymes of tetrahydromethanopterin (H4MPT)-dependent formaldehyde oxidation: mch - N(5),N(10)-methenyltetrahydromethanopterin cyclohydrolase (EC 3.5.4.27); mtdB - methylenetetrahydromethanopterin dehydrogenase (EC 1.5.99.9); mptG - beta-ribofuranosylaminobenzene 5′-phosphate synthase; fhcB - formylmethanofuran dehydrogenase subunit B (EC 1.2.99.5); fhcA - formylmethanofuran dehydrogenase subunit A (EC 1.2.99.5); fhcD - formylmethanofuran–tetrahydromethanopterin N-formyltransferase (EC 2.3.1.101) fhcC - formylmethanofuran dehydrogenase subunit C (EC 1.2.99.5); fae - formaldehyde activating enzyme.
Formylmethanofurane dehydrogenases C, A, B and N-formyl transferase (D) were represented by the formylmethanofurane transferase/hydrolase complex (FTR/hydrolase complex) encoded by the fhcCDAB genes (Methylobacterium extorquens–CAX24163.1–CAX24166.1).
Formate oxidation is the terminal stage of the chain of reactions of direct oxidation in aerobic methylotrophic bacteria. Analysis of A. thiophilum genome revealed formate dehydrogenase of this organism to be a NAD+-dependent enzyme, i.e. a NAD-molybdenum-containing formate dehydrogenase encoded by the fdsABGD genes and additionally three copies of the alpha (fdhA) and delta subunit (fdhD) each (Table S1, Supporting Information). Formate dehydrogenase activity was measured as 0.02 μmol min−1 mg−1 protein.
Azospirillum thiophilum was shown to be capable of growth on either exogenic or endogenic CO2, formed during the processes of methanol and formate oxidation. When no carbonate was present in the medium, microaerobic and aerobic growth (independent on aeration) resulted in a 4.5-fold increase in the cell number: for example, anaerobic cultivation with tetrathionate as the terminal electron acceptor resulted in a 3.3-fold increase. Addition of a carbon source (NaHCO3, 0.5 g L−1) caused the 7–8-fold increase in the cell yield, indicating intensified functioning of the Calvin–Benson–Bassham cycle.
The genes encoding enzymes of the serine and ribulose monophosphate cycles, which involve formaldehyde in constructive metabolism, were not identified in A. thiophilum genome.
Central Metabolism
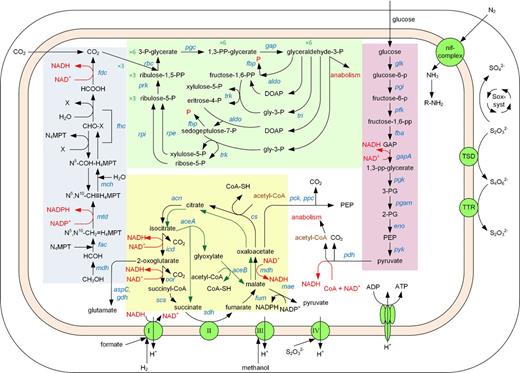
Azospirillum thiophilum utilizes a respiratory metabolism. It does not grow phototrophically and cannot ferment carbohydrates. In line with these observations, a complete set of genes encoding the enzymes for the Embden–Meyerhof-Parnas (EMP) pathway, the tricarboxylic acid (TCA) cycle and glyoxylate cycle (Fig. 8; Table S1, Supporting Information) was found in the genome of A. thiophilum. Pyruvate, a product of the EMP pathway, is then oxidized to acetyl-CoA by the pyruvate dehydrogenase complex. In A. thiophilum, this complex consists of five types of subunits encoded by pdhABC, dld and aceE (Table S1, Supporting Information).
Central metabolism of Azospirillum thiophilum BV-ST. Abbreviations for the genes are given in Table S1 (Supporting Information).
Next, the activities of the enzymes for the TCA and glyoxylate cycles were measured. In A. thiophilum, they were as follows (μmol min−1 mg−1 protein): malate dehydrogenase (NAD), 0.67 ± 0.03; citrate synthase, 0.10 ± 0.01; aconitate hydratase, 0.39 ± 0.02; isocitrate dehydrogenase (NADP), 1.10 ± 0.05; succinate dehydrogenase, 0.58 ± 0.03; fumarate hydratase, 3.00 ± 0.15; isocitrate lyase, 0.11 ± 0.01 and malate synthase, 0.12 ± 0.01.
Azospirillum thiophilum is also capable of oxaloacetate decarboxylation to phosphoenolpyruvate (PEP), since the ppc and pckA genes encoding phosphate-dependent PEP carboxylase and GTP-dependent PEP-carboxykinase were found in its genome. The maeB gene encoding a NADP-dependent malic enzyme, which may produce pyruvate via malate decarboxylation, was also revealed, with the two copies of this gene in the genome. The maeA gene encoding a NAD-dependent malic enzyme was not found.
The analyzed genome also contained genes encoding aspartate aminotransferase (aspC, two copies) catalyzing conversion of aspartate and 2-oxoglutarate to oxaloacetate and glutamate, and gdh coding for glutamate dehydrogenase, which participates in reductive amination of 2-oxoglutarate to glutamate. Thus, exit from the TCA cycle to constructive metabolism may also occur via 2-oxoglutarate.
So some intermediates of the TCA cycle (oxaloacetate, malate and 2-oxoglutarate) may be involved in anabolic processes resulting in formation of phosphoenolpyruvate, pyruvate and amino acids, respectively.
Respiratory electron transport chain
Azospirillum thiophilum genome encodes all major components of the electron transport chain required for energy acquisition via oxidative phosphorylation.
Complex I, NADH:quinone oxidoreductase, is encoded by the genes forming two clusters: nuoABCEFGHJKLMN and nuoDI.
The succinate dehydrogenase complex II is encoded by a four-gene operon, which consists of the sdhC gene encoding the cytochrome b556 subunit, the sdhB gene encoding the iron–sulfur protein, the sdhA gene encoding the flavoprotein subunit and the sdhD gene encoding the hydrophobic membrane anchor protein.
Complex III, ubiquinol-cytochrome c reductase, consists of an iron–sulfur subunit (coded by the rpi1 gene), cytochrome b subunit (coded by the cytB gene) and cytochrome c1 subunit (coded by the cyc1 gene). All these genes are located in a single operon.
Complex IV is represented by five types of oxidases: four cbb3-type subunits (encoded by ccoNOPG), three cox subunits (encoded by cox123), three subunits of cytochrome d ubiquinol oxidase (encoded by cydABCD), two subunits of cytochrome bd (encoded by qxtI and qxtII) and four subunits of cytochrome o ubiquinol oxidase (encoded by cyoABCD).
ADP conversion to ATP occurs via the F-type ATP synthetase encoded by the atpABCDEGH operon, with separately located gene of the F-type H+-transporting ATPase subunit B, atpF.
Phosphates required for ATPase functioning are supplied by an inorganic pyrophosphatase encoded by the ppa gene and polyphosphate kinase encoded by ppk. ppk is located close to the other ATPase genes, while the ppa gene lies outside the cluster.
Terminal oxidoreductases of anaerobic respiratory pathway
Under certain conditions, A. thiophilum was capable of anaerobic respiration using tetrathionate as an electron acceptor (Richard 1977; Barrett and Clark 1987). In line with this observation, genome analysis revealed the presence of an operon, ttrABC, coding for A, B and C subunits of tetrathionate reductase, respectively.
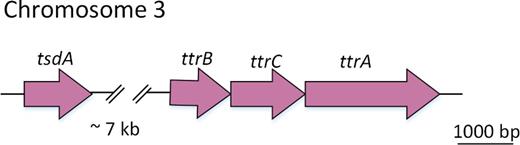
Interestingly, the gene encoding TsdA, responsible for lithotrophic growth in the presence of thiosulfate and its oxidation to tetrathionate, is located not far from the genes of tetrathionate reductase that is involved in anaerobic respiration in the presence of tetrathionate (the distance is ∼7kb, Fig. 9).
Organization of the genes coding for TsdA and tetrathionate reductase.
During heterotrophic anaerobic cultivation of A. thiophilum when tetrathionate was available as a terminal electron acceptor, the cell biomass was increasing. Bacterial growth was accompanied by equimolar reduction of tetrathionate to thiosulfate according to the equation: S4O62− + 2ē = 2 S2O32− (Fig. S1, Supporting Information).
The terminal reductases of anaerobic respiratory pathways are represented by the norCBQDE gene cluster encoding NO reductase (Martinez-Espinosa et al.2007) (Table S1, Supporting Information). The physiological role of predicted NO reductase in A. thiophilum is, however, not confirmed, and till now no information is available on the ability of A. thiophilum to decrease NO release during nitrate reduction.
Nitrogen fixation
The ability of A. thiophilum to fix molecular nitrogen was demonstrated experimentally (Lavrinenko et al.2010). Kwak and Shin reported the results of A. thiophilum genome sequencing when the present article was in preparation. They demonstrated the presence of all genes required for nitrogen fixation (Kwak and Shin 2016). All these genes are located in the first chromosome and form six clusters, being in agreement with our data (Table S1, Supporting Information).
DISCUSSION
Physiological and biochemical investigation, together with the genome analysis revealed that Azospirillum thiophilum possesses a range of metabolic capabilities and is able to utilize diverse types of energy sources and perform constructive metabolism (Fig. 7).
The combined results show that A. thiophilum is a facultative anaerobe and a facultative heterotroph. Several membrane-bound oxidoreductases provide a complete oxidation of organic substances via the TCA cycle with generation of a transmembrane proton gradient further used by ATP synthase to produce ATP. In the presence of external electron acceptors (tetrathionate and, probably, NO) A. thiophilum is capable of anaerobic growth due to anaerobic respiration. Azospirillum thiophilum grows within a broad range of oxygen concentrations, from microaerobic to aerobic. It becomes possible because of the presence of four various terminal oxidases: two different cytochrome c oxidases, cytochrome bd oxidase and quinol o-d oxidase. Most probably, these oxidases have different affinities to O2 and therefore may function under different growth conditions. Ability of the studied organism to grow lithoautotrophically in the presence of H2 or thiosulfate also depends on oxygen concentration. Growth with these substrates is possible under microaerobic conditions only. However, growth with methanol or formate as electron donors under aerobic conditions is also possible. In these cases, the constructive carbon metabolism (anabolism) of A. thiophilum is provided by the enzymes of the Calvin–Benson–Bassham cycle, with both exogenous CO2, and CO2 produced in intracellular oxidation from formate or methanol, being used as carbon sources. Methanol is oxidized to formaldehyde by methanol dehydrogenase, while the latter is oxidized to formate via the tetrahydromethanopterine pathway. Formate dehydrogenases then produce CO2 for the Calvin–Benson–Bassham cycle.
Azospirillum thiophilum inhabits oil and gas bearing region near Krasnodar. This region is characterized by the methane yield on the surface. Thus, methane-oxidizing organisms are most probably present there, and their metabolism results in formation of methanol, formate and formaldehyde. Genes coding for enzymes of methanol oxidation were also found in the genomes of two other sequenced Azospirillum species, A. lipoferum and A. humicireducens, but no experimental data on their ability to oxidize methanol is available at the moment.
Similar to other members of the genus Azospirillum, A. thiophilum is a diazotroph, possessing a complete set of genes involved in N2 fixation.
Based on the obtained information, it can be concluded that the integrated implication of genomic, physiological and biochemical approaches made it possible to reveal a diversity of metabolic pathways in a newly described alphaproteobacterium A. thiophilum, which is important to assess a potential role of these bacteria in environmental ecosystems.
SUPPLEMENTARY DATA
The authors would like to express sincere gratitude to Alexey Fomenkov, Tamas Vincze, Brian P. Anton and Richard J. Roberts for their help with genome sequencing.
FUNDING
This work was supported by [grant ].
Conflict of interest. None declared.
REFERENCES